Mapping the metastable
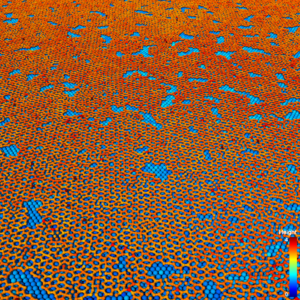
A snapshot from a molecular dynamics simulation showing the growth of a 2D silicene layer (orange) on iridium substrate (blue). (Image: Subramanian Sankaranarayanan, Argonne National Laboratory.)
Materials comprise the technology we use. The number of unknown materials, however, stretches far beyond those currently available. It’s not enough to find those unidentified compounds; scientists also want to predict their capabilities. Today’s supercomputers and advanced algorithms enable calculating those properties by simulating atoms’ arrangement and interactions.
“Atomistic simulations have been around for a while now – several decades,” says Subramanian Sankaranarayanan, leader of Argonne National Laboratory’s theory and modeling group. “But with the recent developments in computational resources we are able to actually make a lot of useful predictions that are accelerating materials discovery and design.”
Even with these advances, scientists still encounter roadblocks. “There are at least a couple of challenges, and one of them is related to the kind of length scales that are accessible,” Sankaranarayanan explains. “There are limitations to the size that you can model with these kinds of simulations because, in principle, they’re accounting for interactions between each atom or molecule.”
Various approximations speed up simulations but not enough to model an entire device and each atomistic interaction. This brings in the element of time. “These simulations are typically used to understand how systems evolve,” Sankaranarayanan says, but they capture nanoseconds or maybe microseconds of interplay, depending on the kind of calculation and the computer architecture.
High-performance computers keep adding processors to produce more speed, but the timing inside the architecture remains about the same. “The clock speeds and bandwidths are not changing a whole lot,” Sankaranarayanan says. “So you’re still limited in the timescale that you can access with these simulations.”
Nonetheless, scientists can easily simulate a few million atoms, which usually translates into tens of nanometers of material. That’s still a small scale, Sankaranarayanan says, but “large compared to what we were able to do even a decade back.” With a coarser-grained simulation – perhaps examining interactions between molecules instead of atoms – scales can increase to microns of material and microseconds, even milliseconds in some cases, of interactions.
Even when a scientist uses a few hundred processors on a high-performance computer, running a simulation still takes a few days to a few weeks. Coarser-grained models let scientists get nearer to the length and time scales that are accessible to experiments.
Most everyday materials are stable, configured at the lowest possible free energy. But metastable compounds – those comprised of atoms in an excited state – can have useful chemical, mechanical or optical characteristics.
“If you look at the thermodynamic landscape, metastable materials are somewhat higher in energy,” trapped in a condition that isn’t at the lowest energy state, Sankaranarayanan says. “This is a state where if you give it enough time or if you push it a little by supplying a bit of energy, the material will actually go to the most stable place.”
Cubic diamond, a form of carbon, is a metastable material. Carbon’s traditional landscape of materials across a range of pressures and temperatures, its so-called phase diagram, includes graphite and other stable forms but not cubic diamond.
In research published in Nature Communications last year, Sankaranarayanan and his colleagues mapped metastable forms of the ubiquitous element. “Depending on how you arrange carbon atoms, they form a different phase,” he says. “It turned out that there were about a thousand different metastable phases of carbon within a reasonable energy cutoff.”
Using the Argonne Leadership Computing Facility’s Theta machine, Sankaranarayanan’s team computed each metastable phase’s free energy, essentially finding how metastable one form was compared to another. As Sankaranarayanan notes, that would require 225,000 sets of calculations to model each of the 1,000 metastable carbon forms at 15 different temperatures and pressures. “We don’t want to do all of them,” Sankaranarayanan says, “and machine-learning algorithms enable a very fast construction of these kinds of maps.”
Researchers can use such maps to explore the properties of metastable materials or employ inverse design, in which a scientist envisions a particular property and then seeks predicted atomic arrangements that could produce it.
Metastable carbon is just one example of what atomistic simulation might teach us about materials. For instance, Sankaranarayanan and his colleagues studied neodymium nickelate – a combination of neodymium, nickel and oxygen – for microelectronics applications based on the location of added hydrogen. “In principle, this could act like artificial intelligence built into microelectronic hardware,” he says.
Water also makes an interesting material for atomistic simulation. “People are excited about different phases of water, understanding their nature on Earth versus some other planets, like Jupiter or Saturn, or extra-terrestrial objects,” Sankaranarayanan says.
Really, scientists could study an endless list of materials this way. “If you take a possible arrangement of atoms for any given material in its most stable state, you can change that arrangement to drive it to a metastable state and see what new properties you would get,” Sankaranarayanan says. “If that property is desired, then the key is being able to make a prediction of how these atoms would be arranged.”
After that, simulations and experimentation work together. A simulation can reveal the conditions needed to create a new metastable material. Experimentalists can try to actually make it. “This is a way to access these metastable phases and make them reliably,” Sankaranarayanan says.
Next, he plans to explore tribology, essentially the study of friction. The results could lead to many new materials, including lubricants. “How do you design these lubricants for, say, automobile engines or other applications where traditional lubricants will actually fail? Can you make new kinds of solid lubricants that will have a better mechanical property?”
Although Sankaranarayanan can’t answer those questions yet, he believes metastable materials will be part of the solution. Like his work on carbon, creating metaphase diagrams will point the way to many new substances that could be used for lubrication in extreme environments. As he says, “Constructing these metaphase diagrams, these maps, is just the beginning.”
About the Author
Mike May has worked as a full-time freelancer since 1998, covering topics ranging from biotech and drug discovery to information technology and optics. Before that, he worked for seven years as an associate editor at American Scientist. He earned an M.S. in biological engineering from the University of Connecticut and a Ph.D. in neurobiology and behavior from Cornell University.





You must be logged in to post a comment.