Back to the hydrogen future
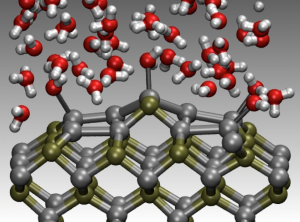
Liquid water molecules binding to a surface in a promising photoactive system, called InP, for generating hydrogen using sunlight and water. (Brandon Wood, Lawrence Livermore National Laboratory.)
As a Massachusetts Institute of Technology (MIT) graduate student researching next-generation energy technologies, Brandon Wood recalls getting pumped when he heard President George W. Bush’s 2003 State of the Union address.
The president envisioned Americans soon fueling-up their cars with hydrogen.
“It was oversold and under-delivered,” says Wood, a Department of Energy Computational Science Graduate Fellowship (DOE CSGF) recipient from 2003 to 2007. “The technological challenges are much greater than people thought.”
A decade of trial and error has revealed that realizing the hydrogen highway dream requires starting very, very small.
“We have to understand hydrogen’s atomic-level interactions,” says Wood, now a research scientist in the Quantum Simulations Group at Lawrence Livermore National Laboratory.
At LLNL he’s doing just that: applying the world’s most sophisticated, fundamental molecular dynamics codes on America’s leading supercomputers to model hydrogen’s reaction kinetics.
Wood has just started as the principal investigator on a $1.2 million DOE Office of Energy Efficiency and Renewable Energy (EERE) project. He leads a team of colleagues from LLNL, Sandia National Laboratories and universities to develop an efficient, on-board chemical-based hydrogen storage material.
“The research is essentially revisiting what I did” as a CSGF recipient, says Wood, who’s now unable to remember the exact title of his Ph.D. thesis.
Hydrogen-powered buses in several North American cities use hydrogen carried in pressurized cryogenically cooled tanks, but those are too bulky and heavy for widespread use in passenger cars.
“The long-term goal is still chemical-based materials storage,” Wood says. “It’s like a sponge that absorbs hydrogen in a solid matrix, giving it a much higher density for storage.”
The as-yet unmet challenge is that the ideal chemical storage material must fully bind hydrogen at ambient environmental temperature and higher-than-ambient pressure, as when fueling a vehicle, but must also readily release the hydrogen at slightly elevated operating temperatures.
During the past decade, Sandia scientists led the development of a list of potential low-molecular-weight absorbent metal-hydride storage materials.
Looks great on paper
“On paper everything looks great; they should work,” Wood says. “But they don’t work as advertised. There’s growing consensus at this point that the problem is in the kinetics.”
That consensus is validated by Wood’s research in the July 2014 issue of the Journal of Physical Chemistry C. The work described in the paper followed up on fundamental discoveries from Wood’s DOE CSGF research and helped to clinch his current EERE funding, he says.
The paper, written with EERE project-team member and University of Georgia theorist Mei-Yin Chou, identified a critical atomic-level Achilles’ heel in metal hydrides.
In the study, Wood’s team used ab initio (starting from the beginning) quantum mechanical simulations, run on supercomputers at DOE’s National Energy Research Scientific Computing Center and LLNL, to reveal the atomic-level dynamics of sodium alanate.
Sodium alanate is a prototypical metal hydride. Its hydrogen storage capacity is too low for practical use but it’s one of the scant candidate materials whose reaction kinetics can be accelerated.
The modeling discovered critical atomic-level changes during hydrogenation and dehydrogenation that alter the reaction dynamics. In sodium alanate, these changes accelerate absorption and release; however, Wood thinks that other metal hydrides don’t exhibit this same benefit, limiting their performance.
“As the hydrogen is pulled out, the remaining matrix transforms into a different phase; the atoms rearrange,” Wood says. “You need to understand all the atomic rearrangements and how these affect the energy barriers of reactions.”
In the upcoming EERE-funded research, Wood says, “we’re looking at one of the materials that looks perfect on paper but doesn’t work in real life: magnesium borohydride.”
The research will be the first to address this hydrogen storage conundrum using a sophisticated multiscale model integrating nanoscale ab initio calculations with mesoscale codes designed to describe phase changes. LLNL and University of Michigan researchers initially developed the codes to simulate lithium-ion battery electrodes.
Wood hopes that “if we can understand the kinetics issues and how they are connected to atomic rearrangements, we can come up with some strategies for actually fixing it – for instance, by stabilizing alternate phases.”
The modeling is forecast to use more than 80 million processor hours on one of LLNL’s leadership-class supercomputers over the project’s three years.
The strategies will give the team’s experimentalists, Sandia scientists Vitalie Stavila and Leonard Klebanoff (with whom Wood brainstormed over lunches for six months in developing the proposal) direction for synthesizing new, more efficient, doped nanoparticle metal hydrides.
Wood is a fan of Slavic literature; he speaks Russian from two years spent in Siberia during a Mormon mission. –A decade into his hydrogen career, he can relate to Leo Tolstoy’s observation: “The two most powerful warriors are patience and time.”
Wood doesn’t expect to immediately solve hydrogen storage and production problems but says, “If we can diagnose the issues, that is a huge step forward.”
About the Author
Jacob Berkowitz is a science writer and author. His latest book is The Stardust Revolution: The New Story of Our Origin in the Stars.




