Quantum gold
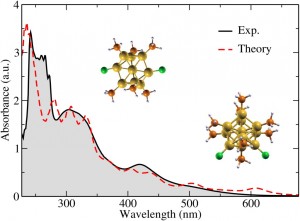
A comparison depicting agreement between the computed optical spectrum (dashed red line) with the ultraviolet-visible light spectrum, as measured in experiments (black line), for a mixed ligand-protected 11-atom and a nine-atom gold nanoclusters. Models of the nanoclusters, with ligand atoms attached, are elsewhere in the illustration. (Yan Li, Brookhaven National Laboratory.)
Li is working with graduate students on detailed theoretical studies to see what factors govern the enhanced hydrogen evolution: nanoparticle size or shape, chemical bonds with the substrate, or the modified electronic structure at the nanoparticle-substrate interface.
As a first step, Li used time-dependent DFT (TDDFT), a generalization of DFT, to calculate the nanoparticles’ light absorption properties.
TDDFT is necessary, Li says, because standard DFT can’t describe the excited electron structures. Like shifting a car into a higher gear, light kicks some electrons in the nanoparticles’ atoms into empty higher energy levels.
Using TDDFT, researchers can calculate the energy and strength of these optical transitions. The method lets them compare the nanoparticles’ computed ultraviolet-visible absorption spectra with spectra measured in experiments. Calculations also can reveal information not readily accessible from experiments, such as the nature of the electronic states involved in optical transitions.
The Stony Brook group, based on its analyses, suggested nanoparticles comprised of either nine or 11 gold atoms dominated the prepared samples. Li computed the corresponding structures and optical spectra of each and compared them with experimental results. “They agree quite well,” Li says. “That gives us some confidence that these are the dominating (particle sizes) in these experiments. The next stage is to look at how they interact with the substrate” to enhance hydrogen evolution so drastically.
Li and a graduate student are working on that problem with support from a Stony Brook seed grant. Eric Isaacs, a Department of Energy Computational Science Graduate Fellowship recipient from Columbia University, contributed to the project while on a practicum this summer.
Meanwhile, Li is looking at other, more complex nanostructures in collaboration with chemist Jonathan Owen and materials scientist Simon Billinge, both at Columbia. Owen’s group synthesized nanoparticles of cadmium-selenide, a material considered a good candidate for photovoltaics. They gauged the nanoparticles’ optical and infrared properties and, with help from BNL scientists, tracked how X-rays bounced off them. Billinge’s group then used data from the X-ray measurements to fit the nanoparticles’ structures.
However, such fitting procedures don’t consider the structures’ energetic stability, so the proposed solutions may not always be physically stable, Li says. And for complex nanostructures, fitting sometimes provides multiple solutions, each agreeing with X-ray measurements to a similar degree but providing few clues about which is the correct answer.
Li is performing DFT calculations to determine whether the proposed structures are physically real and stable. “We want to find out the exact structures of these nanoparticles, as well as how different structures and shapes influence optical properties,” Li says. Eventually, researchers could improve or modify the recipe for synthesizing particles to produce good candidates for photovoltaic materials.
But the models must account for ligands, molecules that attach to the nanoparticle surface, maintaining the surface’s semiconductor-like electronic structure. In the X-ray measurements, signals from the heavier elements cadmium and selenium overshadowed signals from the light atoms comprising the ligands, such as hydrogen, carbon, nitrogen and oxygen. As a result, the measurements tell researchers little about how the ligands bind to the nanoparticle surfaces.
By combining every datum the experiments collected with knowledge from scientific literature, Li plans to reduce the modeling configuration space and validate various stable structures she’s calculated with DFT.
The research tests Billinge’s complex modeling approach, which is based on the assertion that nanostructures’ complex nature makes them difficult to predict with a single technique – in this case, X-ray diffraction. Instead, it takes data from multiple experiments and techniques combined with theory to get at their true geometry.
So far, Owen and Li have identified the structure motif for the smallest cluster the Columbia group synthesized. Results are nearly ready for publication.
“It’s quite exciting,” Li adds. “If it works out we’ll have a methodology we can apply to similar, but bigger and more complex structures.”
It’s another example of the collaboration Li develops with scientists working at the lab bench. “It really takes frequent discussions to make sure we are consistent with each other – that what we compute is actually relevant and can help to understand and improve experiments.”
About the Author
The author is a former Krell Institute science writer.




